Using TophatCufflinks to analyze RNAseq data. MRNA rRNA miRNA converting in some way to DNA and sequencing on a massively parallel sequencing technology such as Illumina Hiseq.

Rna Seq Course Alignment Using Tophat Old Youtube
One of CBSU BioHPC Lab workstations has been allocated for your workshop exercise.
. The analysis in this tutorial is typical of experiments in eukaryotic species with high-quality genomes and genome annotation available. This tutorial will focus on doing a 2 condition 1 replicate transcriptome analysis in mouse. To find junctions with TopHat youll first need to install a Bowtie index for the organism in your RNA-Seq experiment.
RNA-Seq Tutorials Tutorial 1 RNA-Seq experiment design and analysis Instruction on individual software will be provided in other tutorials Tutorial 2 Hands-on using TopHat and Cufflinks in Galaxy Tutorial 3 Advanced RNA-Seq Analysis topics. Tophat Incorporating Illumina RNAseq into AUGUSTUS with TophatThis document describes a method for structurally annotating a genome based on deep sequencing of a transcriptome RNA-Seq. If theres no index for your organism its easy to build one yourself.
RNA Analysis Tophat and set the parameters as follows. Click on the multiple datasets icon and select all six of the forward FASTQ files ending in 1fastq. Bowtie1 is an ultrafast memory-effi cient aligner for large sets of short reads.
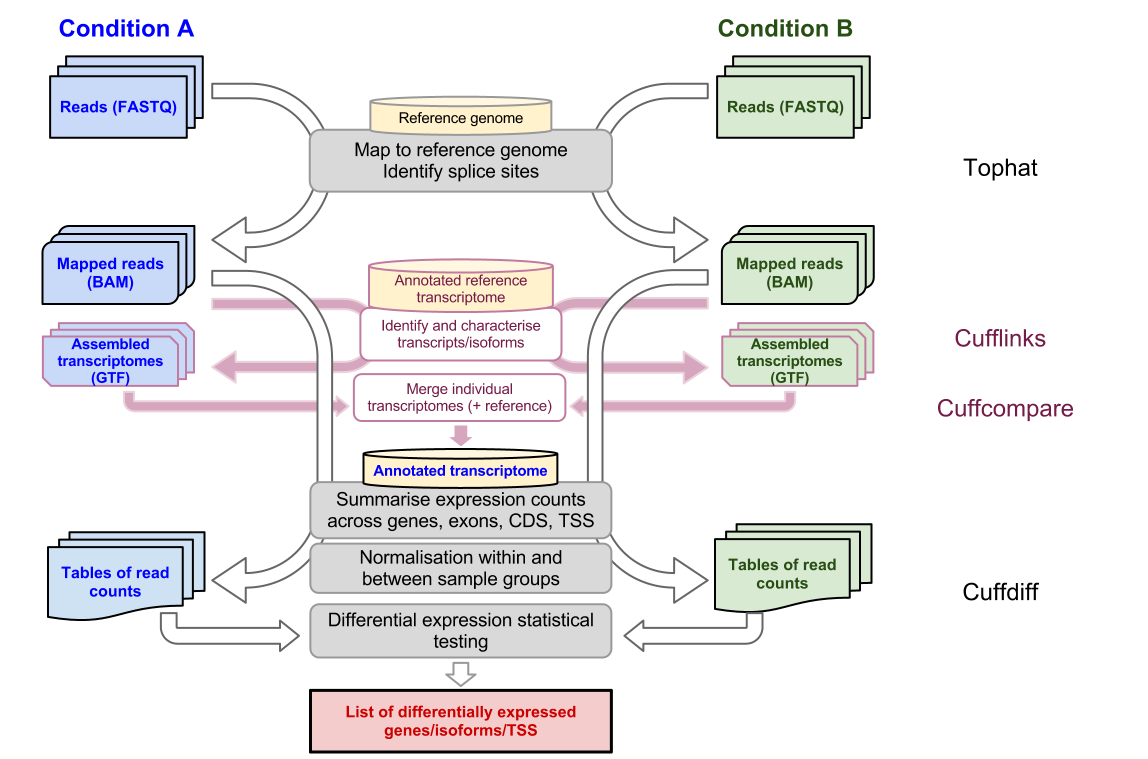
Cufflinks also includes Cuffdiff which accepts the reads assembled from two or more biological conditions and analyzes their differential expression of genes. Some of the applications used in RNA sequencing analysis are the following. RNA-seq as a genomics application is essentially the process of collecting RNA of any type.
Differential expression analysis with limma-voom is covered in an accompanying tutorial RNA-seq counts to genes. Type wq to save and quit vi. Go to an empty line with you cursor and copy paste the new RNA_HOME and PATH commands into the file.
This practical will introduce some popular tools for basic processing of RNA-seq data. Line 3 begins with a character and is optionally followed by the same sequence identifier and any description again. This tutorial from 2017 covers the TopHat aligner.
A Beginners guide to the DESeq2 package 3 RNASeq data preprocessing An RNASeq experiment data analysis starts with FASTQ les obtained as the output of the sequencing runs. In the left tool panel menu under NGS Analysis select NGS. Critically the number of short reads generated for a particular RNA is assumed to be proportional to the.
Httpcbsutccornelleduww1sessionaspxwid9sid12 Please consult the PDF file with instructions on how to access and use the Lab workstations for the. Transcriptome analysis via RNA-Seq. This is a practical hands-on tutorial designed to give participants experience with RNA-Seq data analysis using Tophat Cufflinks and CummRbund in Galaxy.
- Count-based di erential expression analysis of RNA sequencing data using R and Bioconductor 2013 Love et. If you have Bowtie 2 installed and want to use it with Tophat v20 or later you must create Bowtie 2 indexes for your. Getting started The main goal of this activity is to go through a standard method to obtain gene expression values and perform differential gene expression analysis from an RNA.
RNA-seq transcriptome sequencing is a very powerful method for transcriptomic studies that enables quantification of transcript levels as well as discovery of novel transcripts and transcript isoforms. We recommend that you watch the video Aligning RNA-seq reads to reference genome instead which covers t. Everyone should have a BioHPC account to access the computer.
TopHat is a fast splice junction mapper for RNA-Seq reads. TopHat2 uses using Bowtie to align RNA-Seq reads to mammalian-sized genomes and then analyzes the mapping results to identify splice junctions between exons. HISAT2 is the descendent of TopHat one of the first widely-used aligners but alte rna tive mappers could be used such as STAR.
Prepare the working directory. Press the esc key to exit insert mode. RNA-seq Read Mapping with TOPHAT and STAR.
A set of lectures in the Deep Sequencing Data Processing and Analysis module will cover the basic steps and popular pipelines to analyze RNA-seq and ChIP-seq data going from the raw data to gene lists to figures. Is this single-end or paired-end data. Tophat is a splicing aware aligner so we can map transcripts to the genome.
If you would like to learn more about how to use vi try this tutorialgame. Configure the package specifying the install path and the library dependencies as needed. There are several types of RNA-Seq.
To understand the basics of RNA-Seq data how to use RNA-Seq for different objectives and to familiarize yourself with some standard software packages for such analysis. To install TopHat from source package unpack the tarball and change directory to the package directory as follows. This should be correspond to every second file.
The requirements for aligning this type of data is slightly different from eg. These lectures also cover UNIXLinux commands and some programming elements of R a popular freely available statistical software. Paired-end as individual datasets RNA-Seq FASTQ file forward reads.
Much of Galaxy -related features described in this section have been developed by. This tutorial is inspired by an exceptional RNA seq course at the Weill Cornell Medical College compiled by Friederike Dündar Luce Skrabanek and Paul Zumbo and by tutorials produced by Björn Grüning bgruening for Freiburg Galaxy instance. In this tutorial well map reads from an RNA-seq study in Drosophila melanogaster to the reference genome using tophat.
Line 2 is the raw sequence letters. Press the i key to enter insert mode. The guide below was adapted from a description of the method we initially developed for and applied in the RNA-Seq based Genome Annotation Assessment.
TopHat is designed to align RNA-seq reads to a reference genome while Cufflinks assembles these mapped reads into possible transcripts and then generates a final transcriptome assembly. The allocations are listed on the workshop exercise web page. Line 1 begins with a character and is followed by a sequence identifier and an optional description like a FASTA title line.
Find out the name of the computer that has been reserved for you httpscbsutccornelleduwwmachinesaspxi88. At the very end we can compare these results to the results we got from mapping directly to the. The user ID is normally your Cornell NetID.
Press the key to enter command mode. The Bowtie site provides pre-built indices for human mouse fruit fly and others. A FASTQ file normally uses four lines per sequence.
This is quite different conceptually to mapping to the transcriptome directly. Transcriptome splice-variantTSSUTR analysis microRNA-Seq etc. It aligns RNA-Seq reads to mammalian-sized genomes using the ultra high-throughput short read aligner Bowtie included in this plugin and then analyzes the mapping results to identify splice junctions between exonsThis plugin runs on Mac OS and 64-bit Linux only it is not supported Windows.
The tutorial here shows how to start from FASTQ data and perform the mapping and counting steps.
Aligning Rna Seq Data Ngs Analysis
Basic Analyses With Tophat Cufflinks Rnaseq Tutorial 1 Documentation
Introduction To Bulk Rnaseq Analysis Bioinformatics Documentation

Reference Based Rnaseq Data Analysis Long

Reference Based Rnaseq Data Analysis Long
Bioinformatics Greifswald Incorporatingrnaseq Tophat
0 comments
Post a Comment